Abstract
Introduction
Transfusions with red blood cells (RBC) and platelets (PLT) are essential in treatment of patients with AML. There is a paucity of data regarding transfusion utilization among patients receiving induction chemotherapy compared to non-intensive therapy such as hypomethylating agents. Transfusion support is integral to management of AML and can add significant cost to management of AML. We sought to characterize transfusion utilization among AML patients undergoing initial therapy.
Methods
We examined 136 patients at Weill Cornell Medicine Leukemia program who had a diagnosis of AML and achieved complete remission (CR) from 2013 to 2017. We excluded patients with acute promyelocytic leukemia. We collected RBC and PLT transfusions from diagnosis to CR. We collected age, time from diagnosis to CR, inpatient duration, treatment modality, European Leukemia Network (ELN) classification, history of cardiovascular disease, sepsis or bacteremia during induction, Intensive Care Unit (ICU) admission, and bleeding episodes. Mann-Whitney and Kruskal Wallis (with a Nemenyi post-hoc analysis test) were used to compare the number of transfusions between different age, chemotherapy and length of inpatient stay groups. Multivariable linear regression, after log transformation of RBC and PLT transfusions, was performed against the independent variable age at diagnosis controlling for all other risk factors as described above. All analysis was conducted using R version 3.2.3.
Results
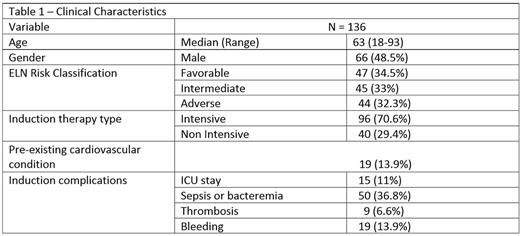
Median age of AML patients was 63 (18-93), and 96 (71%) were treated with intensive induction chemotherapy. Clinical characteristics are summarized in Table 1. Intensive chemotherapy consisted mainly of 7+3, and non-intensive therapy consisted of mainly hypomethylating agents or low dose cytarabine. Median duration to achieve CR was 43 days (14-224), and median inpatient stay was 32 days (0-91). For patients who received induction chemotherapy, median time to CR and hospitalization length of stay were 36 days (14-127) and 32 days (18-78) , and for a non-intensive regimen 81 days (28-224), and 32 days (0-91) respectively. Median RBC utilized from diagnosis to CR was 7 units (0-81), and median PLT was 10 units (0-42).
There was no difference in RBC or PLT transfusion utilization for patients ≥60 compared to <60. (p=0.64, p=0.70 respectively). No significant difference was found for RBC and PLT transfusions between patients receiving intensive vs. non- intensive chemotherapy (p=0.37, p=0.43 respectively). Inpatient stay (≥30 days) was highly significant as a predictor of number of RBC transfusions (p<0.01), but a borderline significant predictor of number of PLT transfusions (p=0.07).
In a multivariate regression analysis for RBC transfusions, length of inpatient stay was the only statistically significant independent predictor (p<0.001, estimated ratio of geometric means: 1.013, 95% Cl (1.007-1.019)). Each additional day of inpatient stay predicts a 1.3% increase in RBC transfusions. For an estimated mean activity-based cost of RBC transfusions at $761/unit, a 10 day increase in inpatient stay with a 13.9% increase in RBC transfusions would result in an increased cost of $1058/patient (calculated based on a median of 10 RBC units). For PLT transfusions, independent predictors were sepsis or bacteremia (p=0.01, estimate: 1.40,95% Cl, (1.079-1.83)), ICU stay (p<0.01, estimate-1.80, 95% Cl, (1.18-2.75)), and lower ELN risk (p<0.05, estimate: 0.73, 95% Cl (0.54-0.99)). PLT transfusions have an acquisition cost of approximately $700 per pathogen-inactivated unit. The average predicted PLT costs for patients with sepsis or bacteremia, ICU admission, or with adverse ELN risk, are $1955/patient, $3930/patient and $1313/patient respectively higher, compared to those without each event or risk factor (calculated based on a median of 7 PLT units).
Conclusion
RBC and PLT transfusions are an integral component of AML treatment. In our analysis, age and induction treatment modality did not influence RBC and PLT utilization. Predictors of increased RBC utilization were increased length of inpatient stay, and for PLT utilization were sepsis or bacteremia, ELN risk, and ICU stay. RBC and PLT transfusions are valuable resources, but not without significant risk; further studies are needed for potentially decreasing utilization safely in treatment of AML patients.
Lee: Amgen: Consultancy; Clinipace: Consultancy; Baxalta: Consultancy; Alexion Pharmaceuticals: Consultancy. Ritchie: Incyte: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Other: Research funding to my institution; Pfizer: Consultancy, Other: Research funding to my institution; NS Pharma: Other: Research funding to my institution; Novartis: Consultancy, Other: Research funding to my institution, and travel, Speakers Bureau; Astellas Pharma: Other: Research funding to my institution; Celgene: Consultancy, Other: Travel, Speakers Bureau. Roboz: AbbVie, Agios, Amgen, Amphivena, Array Biopharma Inc., Astex, AstraZeneca, Celator, Celgene, Clovis Oncology, CTI BioPharma, Genoptix, Immune Pharmaceuticals, Janssen Pharmaceuticals, Juno, MedImmune, MEI Pharma, Novartis, Onconova, Pfizer, Roche Pharmace: Consultancy; Cellectis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal